We present a revolutionary medical device which solves a major female urinary continence problem.
This device, Obtinu, provides instant bladder control.
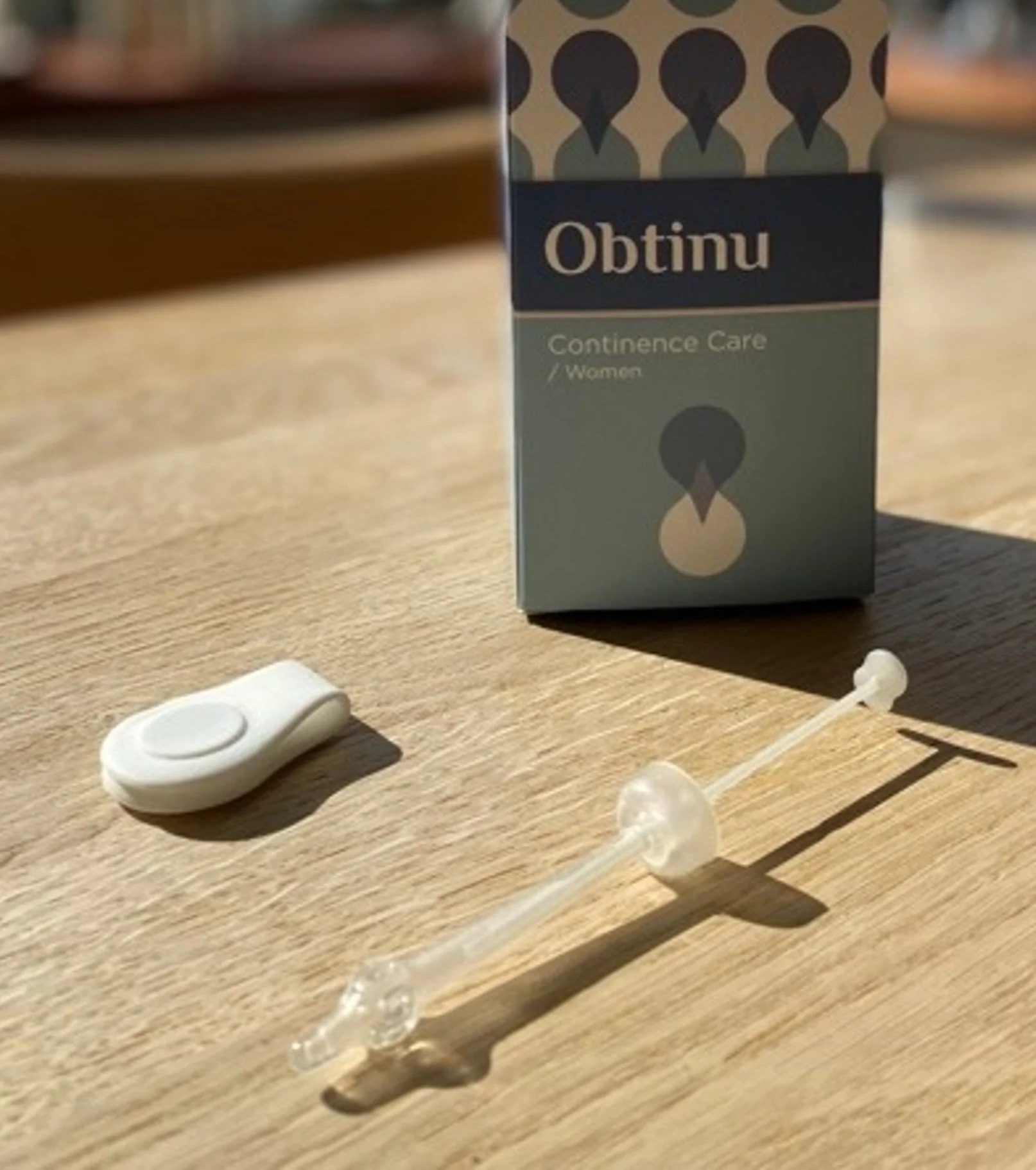
Obtinu is a tissue-like soft, tiny silicone tube that the user herself can insert into the urethra. The tube has a barely visible inner valve, which instantly yields control over all bladder voiding.
Female Stress Incontinence
Each year, 500.000 women undergo mesh surgery due to severe stress incontinence. This has so far been their best available option, despite the risks and the fact that mesh surgery may not work at all. The risk benefit ratio is however so bad, that both the New Zealand health service and the UK NHS have suspended mesh surgery. A better solution is urgently needed!
Three groups of users have successfully tested Obtinu in clinical trials at the Copenhagen University Hospital where doctors conclude that Obtinu is safe and immediately efficient.
Obtinu is protected by two patents, granted in EU, USA and China.